- If it works against coronavirus, mRNA could usher in a revolution in vaccine development.
A nurse prepares a vaccine to be given to a child. A revolutionary mRNA vaccine is hoped to provide an antidote for COVID-19 and against a whole new class of pathogens, or infectious agents, for which no vaccine exists yet. The traditional vaccine industry usually takes time to get themselves organised and develop a shot for a new infectious disease, averaging of 16 years. mRNA could potential speed up this process. In many ways, it digitises vaccine development, using nano technology, to enhance the human body’s own machinery to do exactly what the body does once infected.
Dubai: The world has reopened, but we're all still waiting for a vaccine. A COVID-19 jab is considered the key to lifting social-distancing measures, reopening schools, markets and events around the globe.
Among the front-runners in vaccine development, there are several methods being used: live-attenuated, inactivated, subunit, toxoid, DNA and mRNA.
Today, the most advanced method is the mRNA (messenger ribonucleic acid).
This relatively new platforms remains unproven, but holds much promise. This technology, if it delivers, is hoped to speed up — “revolutionise” — vaccine development.
What do vaccines do?
In general, vaccines “train” and strengthen the body’s immune system to develop resistance against pathogens and illnesses by imitating an infection — to kick up a natural immune response specific to the infectious agent (such as COVID-19 virus).
Why is an mRNA vaccine being dubbed 'revolutionary'?
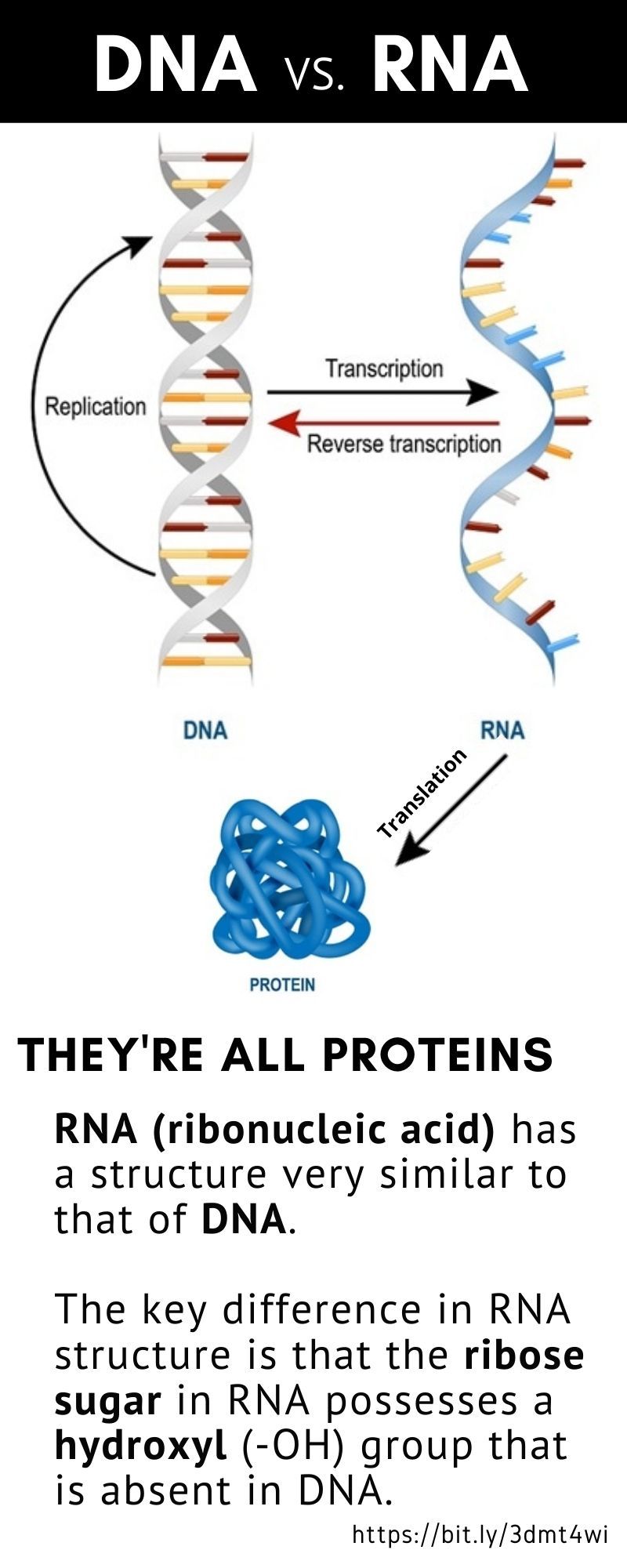
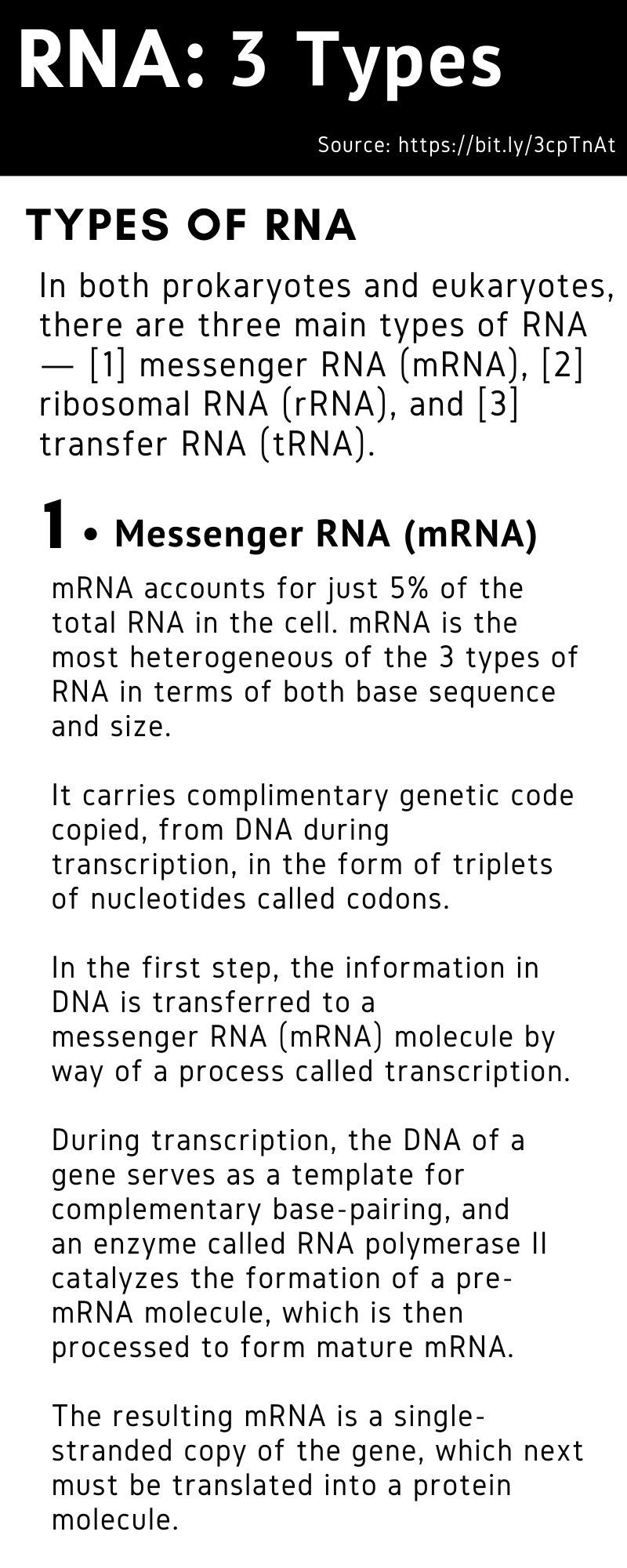
Nucleic acids are the basic building blocks of life. An RNA (ribonucleic acid) vaccine or mRNA (messenger RNA) vaccine is a new type of vaccine.
Among the front-runners in vaccine development, there are several methods being used: live-attenuated, inactivated, subunit, toxoid, DNA and mRNA.
mRNA vaccine is seen as the new hope for the world, now among the most advanced in human trials (Phase-2) to screen a safe and effective COVID-19 shot that will be used on the world's healthy population.
Developing immunity to SARS-CoV-2 would render the virus no worse than the seasonal flu.
There are four phases in vaccine trials, the first 3 being the most crucial.
At the end of the Phase-1 trial of mRNA-1273, involving 45 volunteers, the company said the results were "encouraging", moving it closer to getting the licence for the first mRNA vaccine for human use.
On May 12, Moderna received fast-track approval from the US Food and Drug Administration (FDA) for mRNA-1273. On May 18, the company announced positive interim Phase 1 trial data.
On May 29, the first volunteer under Phase 2 testing of the vaccine was dosed, this time with an estimated 600 participants — including adults below and above age 55.
Phase-3 study (involving thousands of volunteers) begins in July 2020 for Moderna.
Can mRNA vaccines be used to speed up vaccine trials against other infectious diseases?
Yes. mRNA is seen as the advanced biopharma industry's answer to a whole new class of pathogens — or infectious agents — for which no specific vaccines exist yet.
Vaccines are great, and have kept many infectious diseases at bay, saving tens of millions of lives.
However, the traditional vaccine industry usually takes time to get themselves organised and develop a vaccine, typically an average of 16 years.
mRNA speeds up this process. In many ways, it digitises vaccine development to enhance the human body’s own machinery to do exactly what the body does once infected.
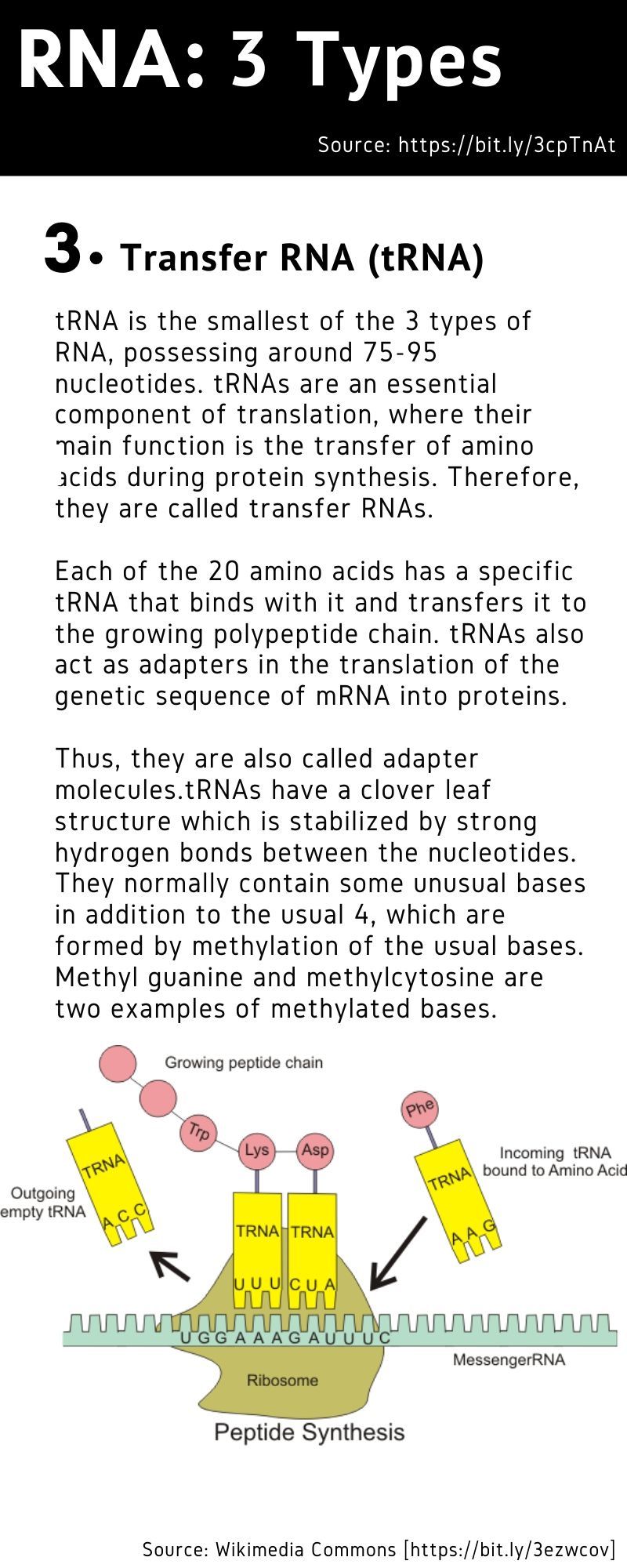
mRNA vaccines are intended to kick up the production of antibodies within the human body, which will bind to fight (disable) potential pathogens when they enter the human body.
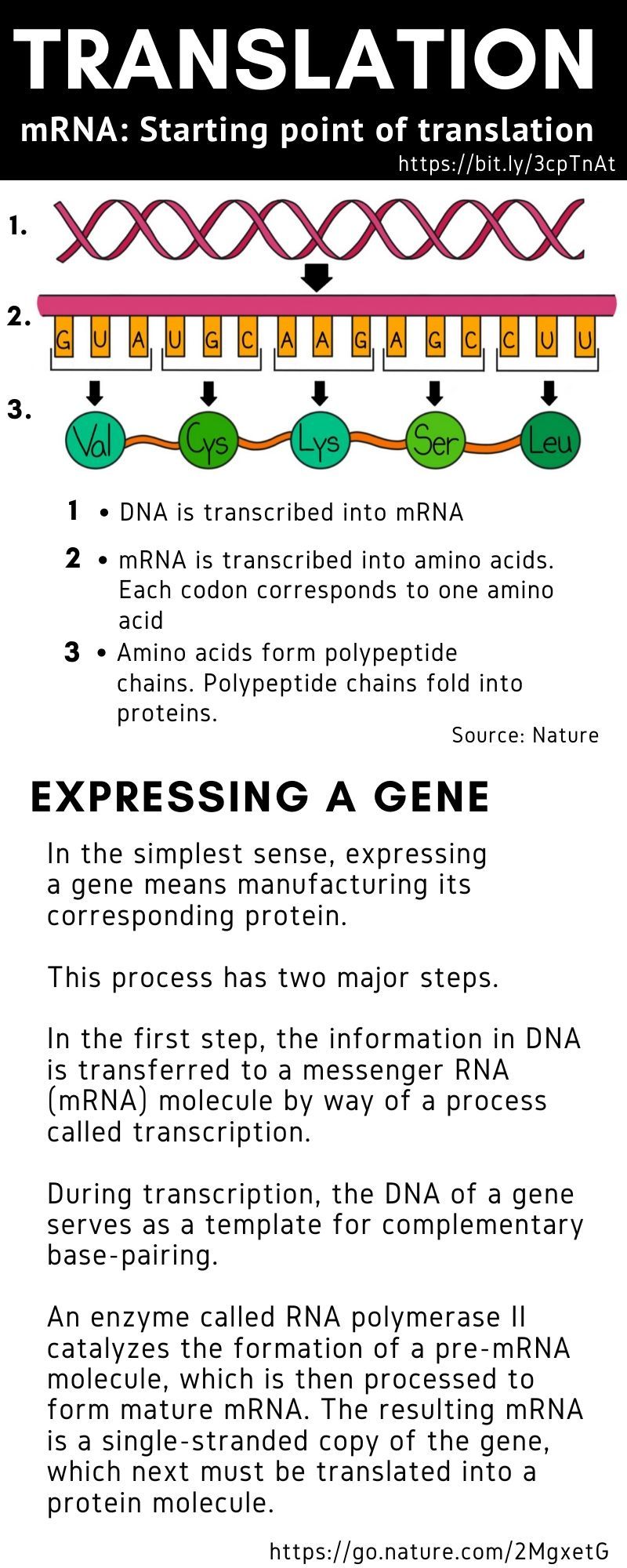
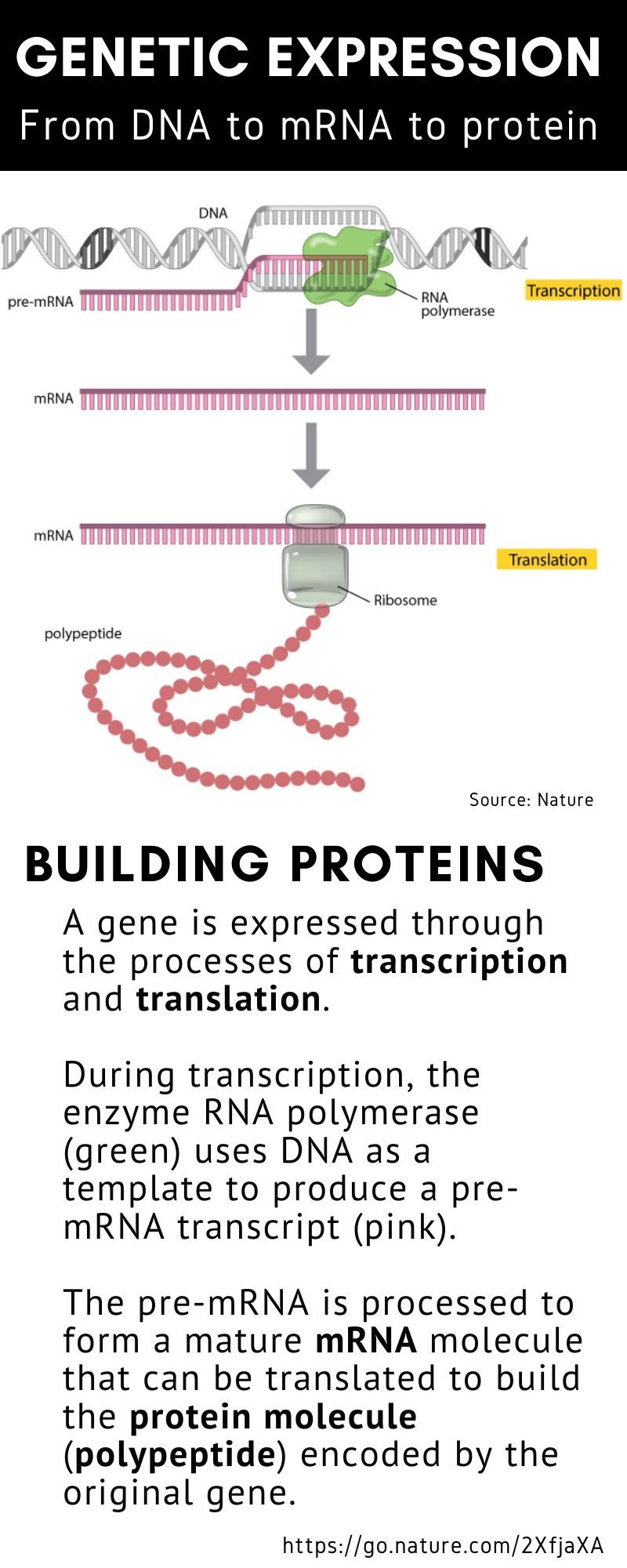
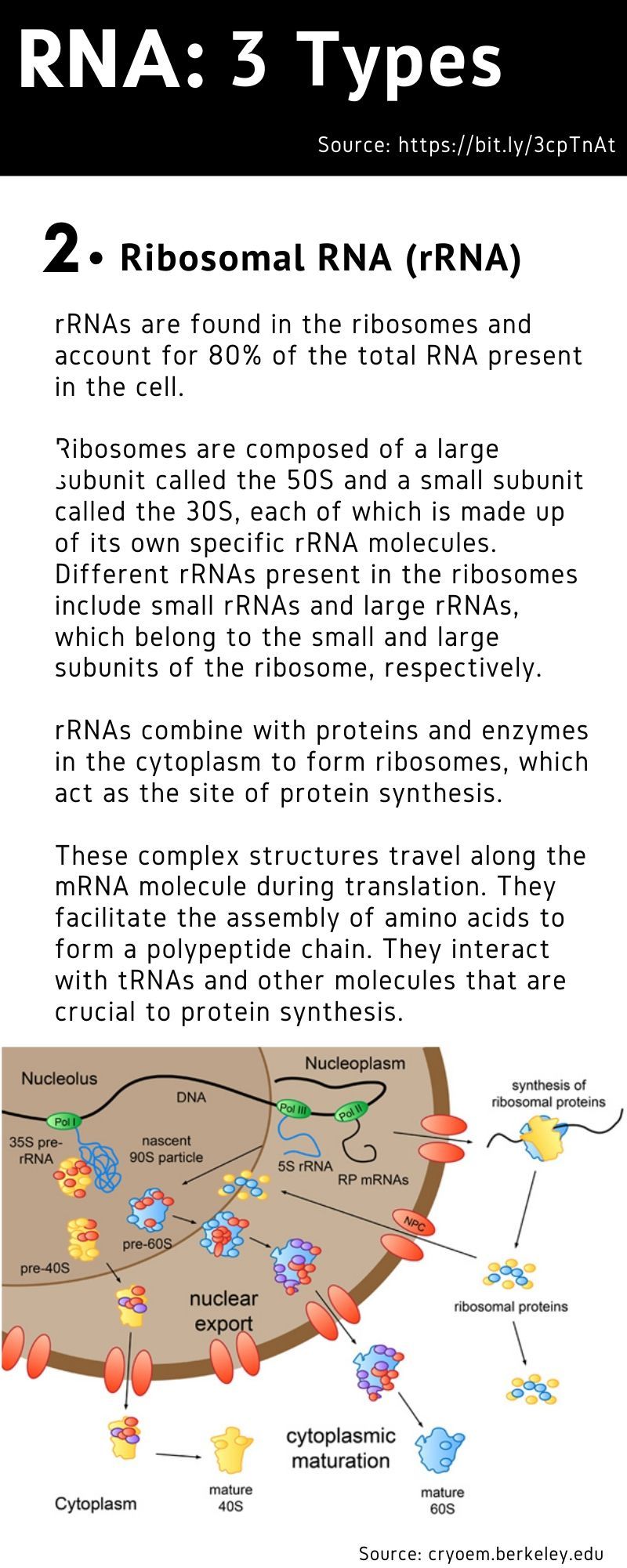
The mRNA sequence codes for antigens (disease-fighting agents), proteins that are identical or resemble those of the pathogen. Upon the delivery of the vaccine into the body, this sequence is translated by the host cells to produce the encoded antigens, which then stimulate the body’s adaptive immune system to produce antibodies against the pathogen.
Basically, an mRNA vaccine provides acquired immunity through an RNA-containing vector, such as lipid nanoparticles.
How does mRNA-vaccine technology work?
The mRNA sequence codes for antigens (disease-fighting agents), or proteins that are identical or resemble those of the pathogen.
Upon the delivery of the vaccine into the body, this sequence is translated by the host cells to produce the encoded antigens, which then stimulate the body’s adaptive immune system to produce antibodies against the pathogen.
Nature reports that the biggest advantage of mRNA technology is rapid manufacturing of vaccines.
In theory, using mRNA as the basis of therapeutics and vaccines is characterised by a flexibility with respect to production and application.
"Any protein can be encoded and expressed by mRNA, in principle enabling the development of prophylactic and therapeutic vaccines fighting diseases as diverse as infections and cancer as well as protein replacement therapies," according to RNAbiology.
How many mRNA vaccines are on trial for COVID-19?
Among the top 10 front-runner candidate vaccines for COVID-19, there are currently two based on mRNA technology: one each by Moderna (US) and another by Pfizer/BioNTech/Fosun (US-Germany-China).
In total, there are 18 mRNA-based candidate vaccines in development globally for COVID-19, according to GlobalData’s Pharma Intelligence Center Drugs Database.
WHAT IS AN ANTIGEN?
In immunology, an antigen is a molecule or molecular structure — such as may be present at the outside of a pathogen — that can be bound to by an antigen-specific antibody or B cell antigen receptor.
The presence of antigens in the body normally triggers an immune response.
When was mRNA first used?
mRNA as a therapeutic was first promoted in 1989 after the development of a broadly applicable in vitro transfection technique. A few years later, mRNA was advocated as a vaccine platform.
It was deemed ideal as it brings together the immunological features of live "attenuated" vaccines such as endogenous antigen expression and T-cell induction — with those of "killed" (or subunit vaccines_ like defined composition and safety.
What's the different between 'attenuated' vs 'inactivated' vaccine?
An attenuated vaccine is a vaccine created by reducing the virulence of a pathogen (disease-causing agent), but still keeping it viable (or "live"). Attenuation takes an infectious agent and alters it so that it becomes (theoretically) harmless or less virulent.
In contrast, an inactivated vaccine (or "killed" vaccine) is a vaccine consisting of virus particles, bacteria, or other pathogens that have been grown in culture and then lose disease-producing capacity.
Examples of inactivated vaccines include: inactivated poliovirus (IPV) vaccine, whole cell pertussis (whooping cough) vaccine, rabies vaccine and the hepatitis A virus vaccine.
Are there mRNA vaccines approved for use by humans for COVID-19?
No. Or not yet. But there are mRNA vaccines on trial as antidote to the coronavirus.
In immunology, an antigen is a molecule or molecular structure — such as may be present at the outside of a pathogen — that can be bound to by an antigen-specific antibody or B cell antigen receptor.
The presence of antigens in the body normally triggers an immune response.
When was mRNA first used?
mRNA as a therapeutic was first promoted in 1989 after the development of a broadly applicable in vitro transfection technique. A few years later, mRNA was advocated as a vaccine platform.
It was deemed ideal as it brings together the immunological features of live "attenuated" vaccines such as endogenous antigen expression and T-cell induction — with those of "killed" (or subunit vaccines_ like defined composition and safety.
What's the different between 'attenuated' vs 'inactivated' vaccine?
An attenuated vaccine is a vaccine created by reducing the virulence of a pathogen (disease-causing agent), but still keeping it viable (or "live"). Attenuation takes an infectious agent and alters it so that it becomes (theoretically) harmless or less virulent.
In contrast, an inactivated vaccine (or "killed" vaccine) is a vaccine consisting of virus particles, bacteria, or other pathogens that have been grown in culture and then lose disease-producing capacity.
Examples of inactivated vaccines include: inactivated poliovirus (IPV) vaccine, whole cell pertussis (whooping cough) vaccine, rabies vaccine and the hepatitis A virus vaccine.
Are there mRNA vaccines approved for use by humans for COVID-19?
No. Or not yet. But there are mRNA vaccines on trial as antidote to the coronavirus.



What are the leading mRNA vaccine candidates?
There are two, among the leading 10:
BNT 162 - Pfizer/BioNTech/Fosun Pharma
mRNA-1273 - Moderna/National Institute of Allergy and Infectious Diseases
What are the advantages of mRNA vaccine over DNA or other traditional ones?
mRNA vaccines offer multiple advantages over DNA vaccines in terms of the following:
1.Mass production
2.Administration
3.SafetymRNA vaccines offer multiple advantages over DNA vaccines RNA vaccines are also thought to have the potential to be used for cancer in addition to infectious diseases.
Multiple companies, including CureVac and Moderna, Pfizer/BioNTech/Fosun work on the development of mRNA vaccines to combat the COVID-19 pandemic.
If the mRNA vaccines pass rigorous trials — proving they're safe and efficacious following trials on thousands of subjects/volunteers — one key advantage hinges on rapidity of manufacture. This is because the process is cell-free and scalable.
In addition, new targets requiring multi-antigen approaches will benefit from the speed in which mRNA can render multiple constructs, according to Nature.
Who will approve the vaccine, and review the trials?
They will go through approvals in different jurisdictions. The Who keeps an online database of all the trials and their status. In the US, the vaccine must be approved the FDA (US Food and Drug Administration).
For Europe, it’s the EMA (European Medicines Agency). Every country has its own drug and vaccine regulatory body who also must approved the vaccine.
Who are the major vaccine developers?
Other major pharma giants in various stages of vaccine development include: AstraZeneca/Oxford GlaxoSmithKline/ Sanofi, Johnson & Johnson/US Biomedical Advanced Research and Development Authority (BARDA). In addition, there are Chinese, Indian, Italian biotech firms — as well as Mexican, Japanese, Danish and Thai vaccine developers, among others.
What are the results of mRNA vaccine trials in human volunteers?
On May 18, 2020, Modern said its vaccine trials showed “early signs” of viral immune response. Moderna injected the first mRNA-based vaccines on human volunteers in March 16, 2020.

What they found: At two lower dose-levels used in the study, levels of antibodies found after getting a second booster shot of the vaccine either equaled or exceeded the levels of antibodies found in patients who had recovered from the virus.
It’s an early sign that an antibody was made and can stop the virus from replicating. The company said that safety profile appeared to be “good”, and the reactions were typical of vaccines. They included injection site pain and redness, as well as temporary fever or chills that quickly go away on their own, said officials.
Meanwhile, Pfizer is also conducting clinical trials in the US and Europe for its BNT162 vaccine against SARS-CoV-2 virus with with German mRNA company BioNTech.







No comments :
Post a Comment